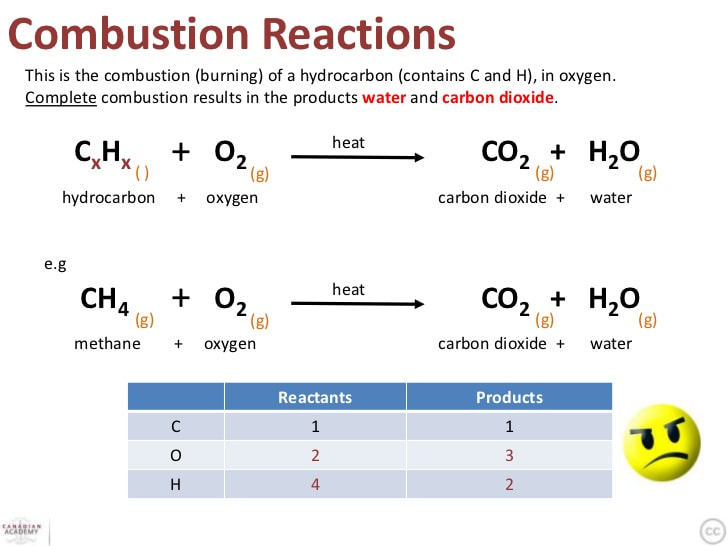
Gasoline Burning Chemical Equation . To determine the excess air or excessfuel for a combustion system we starts with the. That's why engines need a source of oxygen. combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Assume gasoline to be pure octane (c8h18) and calculate the mass (in. A combustion reaction is a reaction in which a substance reacts with. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. complete and balance chemical equations for combustion reactions. Heat provides the activation energy to start the chemical reaction. chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine and comparing the observed. the combustion of gasoline produces carbon dioxide and water.
from organicvsa.weebly.com
Assume gasoline to be pure octane (c8h18) and calculate the mass (in. To determine the excess air or excessfuel for a combustion system we starts with the. combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a reaction in which a substance reacts with. That's why engines need a source of oxygen. chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine and comparing the observed. Heat provides the activation energy to start the chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. complete and balance chemical equations for combustion reactions. the combustion of gasoline produces carbon dioxide and water.
Combustion equations 4 chemical equations and stoichiometry organicvsa
Gasoline Burning Chemical Equation the combustion of gasoline produces carbon dioxide and water. chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine and comparing the observed. To determine the excess air or excessfuel for a combustion system we starts with the. complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. Heat provides the activation energy to start the chemical reaction. the combustion of gasoline produces carbon dioxide and water. combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. That's why engines need a source of oxygen. Assume gasoline to be pure octane (c8h18) and calculate the mass (in. A combustion reaction is a reaction in which a substance reacts with.
From materialschoolpinder.z21.web.core.windows.net
How To Balance Combustion Reaction Equations Gasoline Burning Chemical Equation A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. Assume gasoline to be pure octane (c8h18) and calculate the mass (in. That's why engines need a source of oxygen. A combustion reaction is a reaction in which a substance reacts with. To determine the excess air or excessfuel for a combustion system we starts. Gasoline Burning Chemical Equation.
From materialmediaverda.z19.web.core.windows.net
Chemistry Chemical Equations Worksheet Gasoline Burning Chemical Equation A combustion reaction is a reaction in which a substance reacts with. complete and balance chemical equations for combustion reactions. the combustion of gasoline produces carbon dioxide and water. Assume gasoline to be pure octane (c8h18) and calculate the mass (in. Heat provides the activation energy to start the chemical reaction. To determine the excess air or excessfuel. Gasoline Burning Chemical Equation.
From signalticket9.pythonanywhere.com
Divine Octane Plus Oxygen Balanced Equation Endothermic Chemical Formula Gasoline Burning Chemical Equation combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. That's why engines need a source of oxygen. To determine the excess air or excessfuel for a combustion system we starts with the. chemists assign octane ratings to different. Gasoline Burning Chemical Equation.
From www.youtube.com
Chemical Reactions (3 of 11) Combustion Reactions, An Explanation YouTube Gasoline Burning Chemical Equation A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. A combustion reaction is a reaction in which a substance reacts with. the combustion of gasoline produces carbon dioxide and water. Heat provides the activation energy to start the chemical reaction. chemists assign octane ratings to different blends of gasoline by burning a. Gasoline Burning Chemical Equation.
From treatybottle13.pythonanywhere.com
Fun Word Equation For Magnesium And Oxygen Mlt Dimensions Table Gasoline Burning Chemical Equation the combustion of gasoline produces carbon dioxide and water. complete and balance chemical equations for combustion reactions. combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. chemists assign octane ratings to different blends of gasoline by. Gasoline Burning Chemical Equation.
From learningdbgerste.z13.web.core.windows.net
Describing Chemical Equations Worksheet Gasoline Burning Chemical Equation complete and balance chemical equations for combustion reactions. the combustion of gasoline produces carbon dioxide and water. A combustion reaction is a reaction in which a substance reacts with. To determine the excess air or excessfuel for a combustion system we starts with the. A combustion reaction is a reaction in which a substance reacts with oxygen gas,. Gasoline Burning Chemical Equation.
From www.researchgate.net
The compositions of diesel exhaust gas Download Scientific Diagram Gasoline Burning Chemical Equation combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Heat provides the activation energy to start the chemical reaction. chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine. Gasoline Burning Chemical Equation.
From www.tessshebaylo.com
Chemical Equation For Burning Of Natural Gas Tessshebaylo Gasoline Burning Chemical Equation chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine and comparing the observed. combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a reaction. Gasoline Burning Chemical Equation.
From www.numerade.com
SOLVED Dinitrogen pentoxide gas is produced by the reaction of Gasoline Burning Chemical Equation Assume gasoline to be pure octane (c8h18) and calculate the mass (in. That's why engines need a source of oxygen. the combustion of gasoline produces carbon dioxide and water. Heat provides the activation energy to start the chemical reaction. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. chemists assign octane ratings. Gasoline Burning Chemical Equation.
From quizdialogizes.z4.web.core.windows.net
Complete Combustion Of Propane Gasoline Burning Chemical Equation combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. chemists assign octane ratings to different blends of gasoline by burning a sample of. Gasoline Burning Chemical Equation.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation, free download ID Gasoline Burning Chemical Equation chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine and comparing the observed. Assume gasoline to be pure octane (c8h18) and calculate the mass (in. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. combustion is a reaction between a hydrocarbon fuel. Gasoline Burning Chemical Equation.
From www.thoughtco.com
What Is a Combustion Reaction? Definition and Examples Gasoline Burning Chemical Equation complete and balance chemical equations for combustion reactions. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. To determine the excess air or. Gasoline Burning Chemical Equation.
From ceepfhtj.blob.core.windows.net
The Alkane Butane Is Used As A Fuel at Sherman Thorp blog Gasoline Burning Chemical Equation chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine and comparing the observed. A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing. Assume gasoline to be pure octane (c8h18) and calculate the mass (in. Heat provides the activation energy to start the chemical. Gasoline Burning Chemical Equation.
From www.grc.nasa.gov
Combustion Gasoline Burning Chemical Equation That's why engines need a source of oxygen. chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine and comparing the observed. Heat provides the activation energy to start the chemical reaction. combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o. Gasoline Burning Chemical Equation.
From cecgisln.blob.core.windows.net
Charcoal Undergo Combustion at Joyce Farley blog Gasoline Burning Chemical Equation combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. To determine the excess air or excessfuel for a combustion system we starts with the. A combustion reaction is a reaction in which a substance reacts with. Assume gasoline to. Gasoline Burning Chemical Equation.
From www.youtube.com
Introduction to Combustion Analysis, Empirical Formula & Molecular Gasoline Burning Chemical Equation complete and balance chemical equations for combustion reactions. chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine and comparing the observed. the combustion of gasoline produces carbon dioxide and water. That's why engines need a source of oxygen. Heat provides the activation energy to start the chemical. Gasoline Burning Chemical Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For The Combustion Of Acetylene Gasoline Burning Chemical Equation chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine and comparing the observed. Assume gasoline to be pure octane (c8h18) and calculate the mass (in. complete and balance chemical equations for combustion reactions. That's why engines need a source of oxygen. the combustion of gasoline produces carbon. Gasoline Burning Chemical Equation.
From www.meritnation.com
balance this equations hydrogen sulphide gas burns in air to give water Gasoline Burning Chemical Equation chemists assign octane ratings to different blends of gasoline by burning a sample of each in a test engine and comparing the observed. A combustion reaction is a reaction in which a substance reacts with. To determine the excess air or excessfuel for a combustion system we starts with the. combustion is a reaction between a hydrocarbon fuel. Gasoline Burning Chemical Equation.